




REGENSIFY 增密头发营养补充剂 (REGENSIFY Redensifying Hair Nutrition)
美国 FDA 注册号:19297765412 - 新加坡工厂有在美国 FDA 注册,并符合所有制造标准和审核。
完成 REGENSIFY 护发护理只需四步
- REGENSIFY DHT 阻断洗发水 - 清洁和修复
- REGENSIFY 头发再生护发素 - 恢复和保湿
- REGENSIFY 浓密再生护发精华液 - 加速生长
- REGENSIFY 增密头发营养补充剂 - 强韧和滋养
90 粒胶囊 | 30 天 | 每天 3 粒胶囊
新加坡制造 | GMP 认证 | FDA 注册教师 | 第三方实验室测试 | 采用天然成分制成 | 不含麸质 | 非转基因
研发和制造源自于新加坡
让 REGENSIFY 使你的头发重获浓密
注意:在美国和澳大利亚市场,产品会有 REGENSIFY Redensifying Hair Nutrition 的标签。在新加坡、马来西亚和其他市场,产品标签可能贴有 REGENSIFY AnaGain Nu with DHT Blocker 标签。两者为同一款产品。
富含有专利成分 AnaGain™ Nu、DHT 阻断剂(锯棕榈、海藻提取物)、辅酶 Q10、L-肉碱 L-酒石酸盐、生育三烯酚、三肽胶原蛋白、生物素、角蛋白、维生素 D3、锌和铁配制而成,这是一款高级护发营养补充剂。
配方中的每种成分都因为它们具有临床疗效而经过特别被挑选。REGENSIFY 增密头发营养补充剂 (REGENSIFY Redensifying Hair Nutrition) 是在新加坡的一家 GMP 工厂配制和生产的。该补充剂 100% 不含药物。每批产品都会经过严格的第三方实验室检测,以筛查有毒重金属和微生物限值,来确保可以安全食用。
为什么每天要服用三粒胶囊?
每天服用一到两粒胶囊的配方无法包含所有的必需营养。每天服用四粒胶囊可能过多。每天服用三粒胶囊可以达到合适的平衡,从而使配方能够包含全面的成分,以提供所有的必需营养。在 REGENSIFY,我们坚信使用有科学依据的成分, 无论是化妆品或营养保健品。
每日 3 粒胶囊,喝水送用。无已知副作用。请坚持建议剂量至少 4 至 6 个月,以获得最佳效果。请勿过量或剂量不足。本补充剂适用于所有正在经历头发稀疏和脱发(脱发期间每天超过 100 根头发)的成年男性和女性。您可以餐前或餐后服用,每日分三次服用,或一次性服用三粒胶囊。
注意:如果生病,正在服用任何药物或怀孕期间,请勿服用。如有疑问,请在使用前咨询医生。正在服用华法林治疗期间,若未经过医生建议请勿服用。
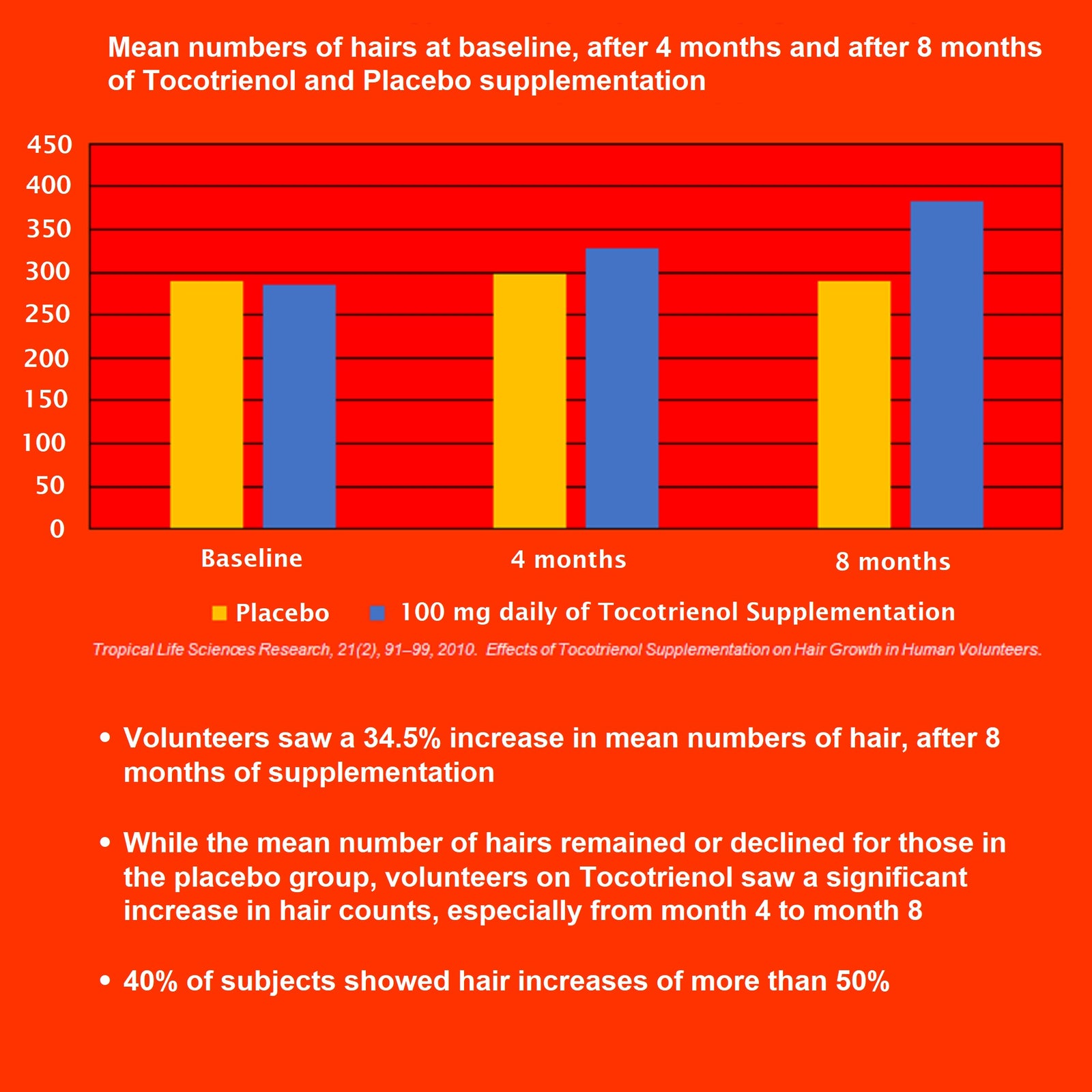
- 维生素 E(生育三烯酚)- 100 毫克
- 生物素 - 675 微克
- 锌(葡萄糖酸锌)- 15 毫克
- 铁(硫酸亚铁)- 15 毫克
- 维生素 D3(胆钙化醇)- 20 微克
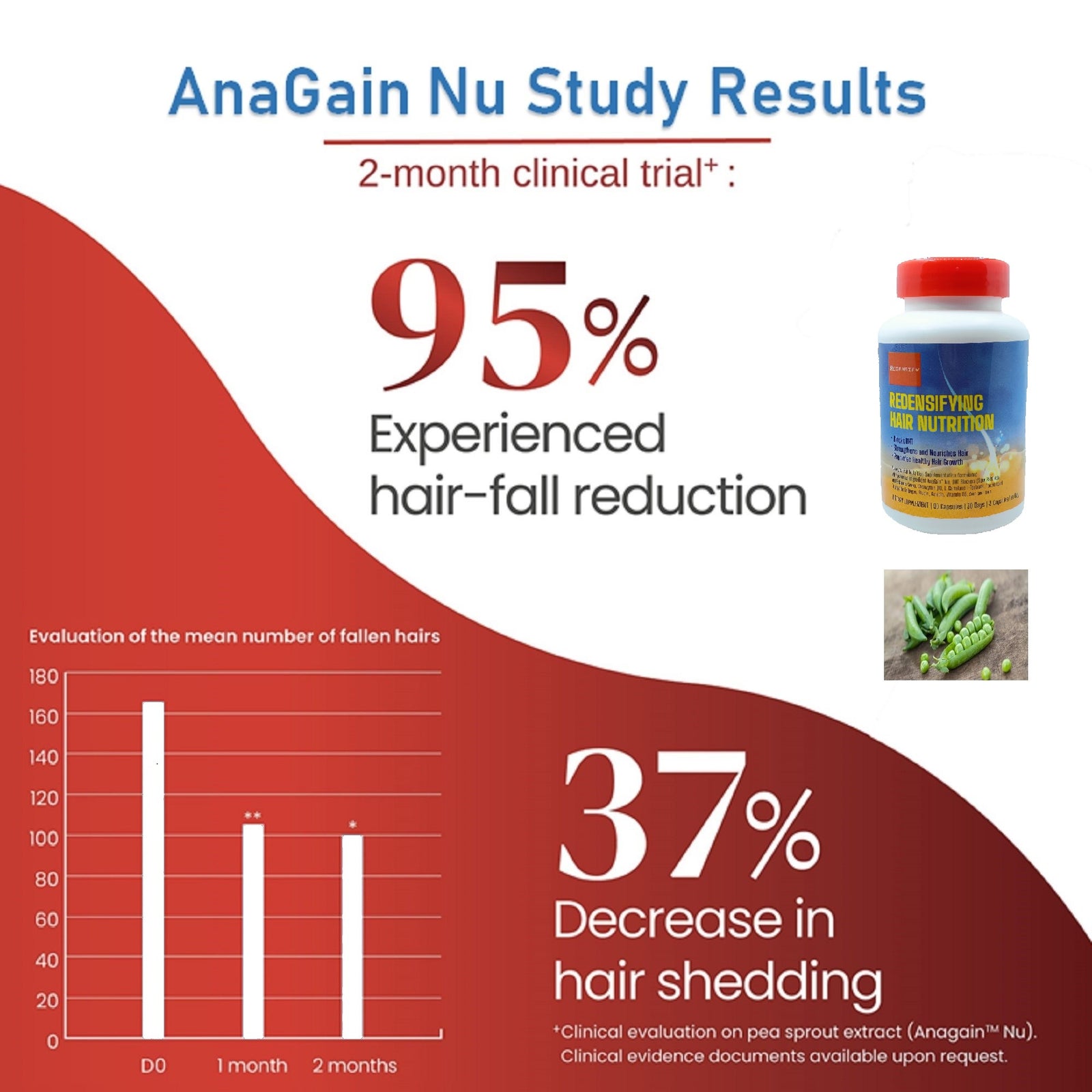
- AnaGain™ Nu(豌豆芽提取物)- 100 毫克
- 锯棕榈提取物(Serona Repens)(果实)- 360 毫克
- 昆布提取物 - 750 毫克
- 辅酶 Q10(泛醌)- 100 毫克
- L-肉碱-L-酒石酸盐 - 150 毫克
- 三肽胶原蛋白(水解海洋胶原蛋白)- 100 毫克
- 角蛋白 - 100 毫克



































































